Protein expression in bacteria is by far the most common method employed to prepare stable isotope enriched protein samples. Escherichia coli is the most popular organism for the expression of recombinant proteins, being a well-understood host with short culture times. E. coli enables straightforward gene manipulation using simple molecular techniques and the cells show good tolerance to deuterium, allowing for cost effective labeling with 13C, 15N and 2H.
Under anaerobic conditions, E. coli can synthesize all 20 essential amino acids employing glucose, acetate and glycerol as their sole source of carbon along with ammonium salts as their sole source of nitrogen. The use of labeled minimal media reagents such as deuterium oxide, 15N ammonium salts, 13C6, D7 or 13C6/D7 labeled glucose allows for the straightforward production of uniformly labeled protein samples.
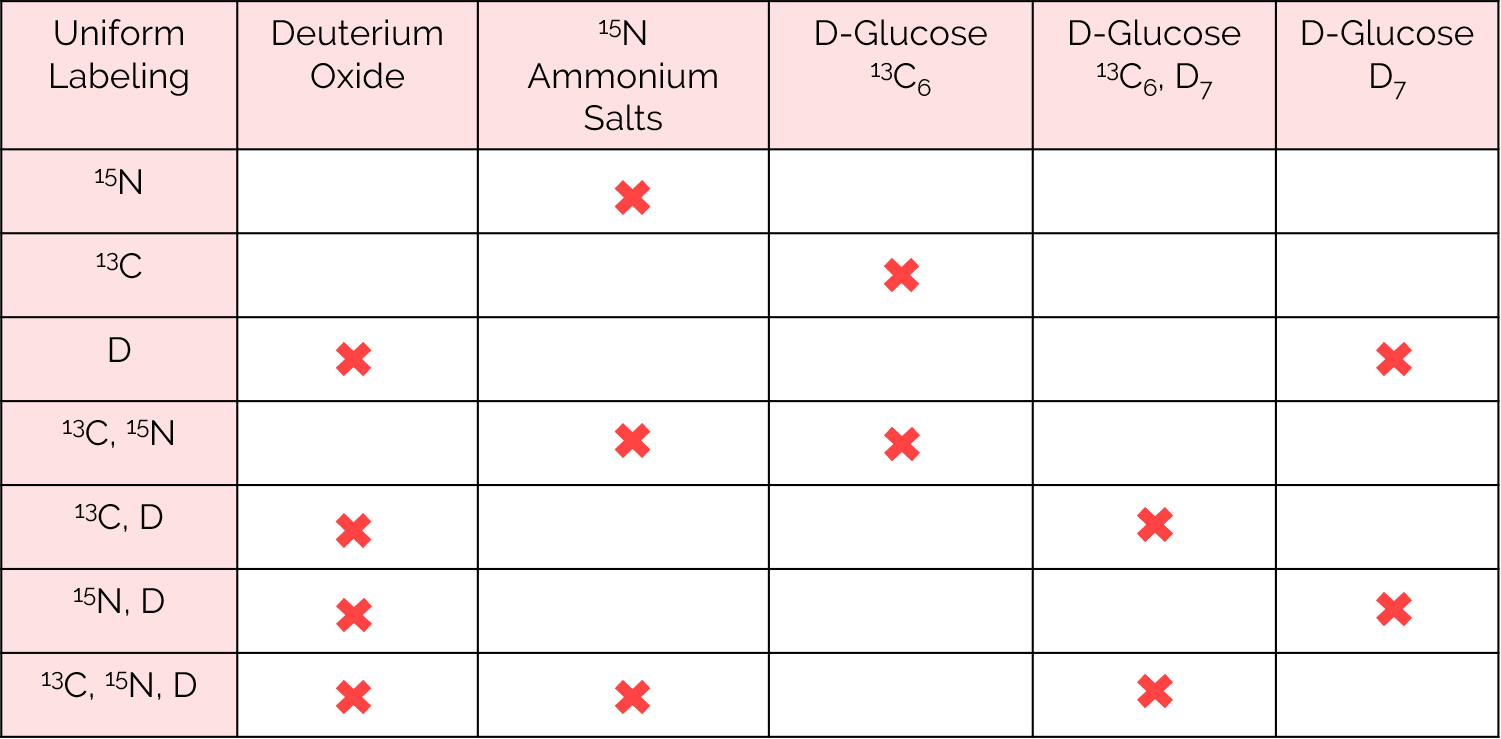
Uniform labeling obtained with the corresponding labeled reagents
ISOGRO is a complex powdered rich media derived from algal lysate and has been developed to enhance protein expression in bacteria. ISOGRO powder is available with various combination of 13C, 15N and 2H labeling and can either be used as a stand-alone media or a supplement to minimal media to improve protein yields.
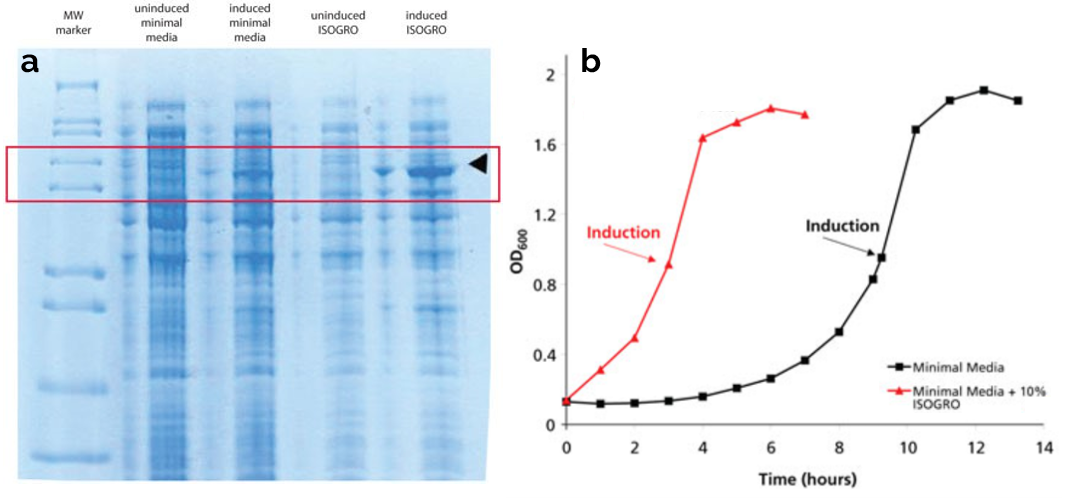
a. SDS-PAGE of p38 growth. Data provided by Dr. Jeffrey W. Peng, Dept. of Chem./Biochem., Univ. of Notre Dame, Indiana, USA. b. Cardiac troponin cTnC(1-89) in pLysS. Cells grown at 37°C in shaker flasks. Red curve is ISOGRO supplemented minimal media and black curve is minimal media alone.
If protein expression cannot be successfully performed using E. coli, other options can be considered, such as insect cell, mammalian cell or cell-free expression systems.

Cortec Isotope is pleased to offer a wide selection of isotopically enriched compounds for protein production in E. coli.
Cortec Isotope proposes to help you label your protein. For inquiry, please contact our labeled protein expression platform.